Regulatory toxicology
Our team will ensure that your product is compliant to the European rules.
Article 3 of Cosmetic Regulation (EC) No 1223/2009 states: "A cosmetic product made available on the market shall be safe for human health when used under normal or reasonably foreseeable conditions of use".
The purpose of pre-assessment safety reports is to establish an initial review of the regulatory compliance and safety of formulations that are still in development or that have been designed for vulnerable target populations (i.e. babies, pregnant or breastfeeding women, etc.)
With these pre-assessment safety reports, Eurosafe guides you on the following matters:
- the decision to undergo specific in vitro or clinical tolerance tests to ensure product safety,
- a precise listing of all tests that may potentially be required, according to anticipated labeling and written usage guidelines,
- potential recommendations for usage precautions to label.
To meet the specific safety challenges and expectations of a market where innovation is central, Eurosafe is able to develop customized evaluation methods specifically adapted to your needs.

The product information file (PIF) includes all information relating to identity, quality, safety, and claims of cosmetic products.
It consists of five parts:
- 1st part: Administrative data (product description, responsible parties, contact persons, etc.)
- 2nd part: Cosmetic product safety report
- 3rd part: Manufacturing and packaging methods (in conformity with the Good Manufacturing Practices ISO 22716)
- 4th part: Claims and Effectiveness
- 5th part: Data on animal experimentation
In addition to creating the PIF, Eurosafe offers the following services:
- Product notifications on the EU Cosmetic Notification Portal
- Regulation analysis and compliance of your formulation
When the product is ready for sale, a safety report is established according to the requirements of Annex I of Cosmetics Regulation (EC) No 1223/2009.
This report, which corresponds to the main component of the Product Information File (PIF), includes two parts:
- Part A - Cosmetic product safety information
- Part B - Cosmetic product safety assessment
The safety assessment, or toxicological risk assessment, is performed on the basis of the cosmetic formulation and the normal or reasonably foreseeable conditions of use.
Eurosafe has a database with toxicological profiles of several thousand cosmetic substances, enabling the evaluation of diverse types of cosmetic products.
In vitro assays
Our laboratory historically specializes in in vitro studies. We offer a portfolio of assays ranging from the well-established to the most cutting-edge. Some assays are regulatory compliant to ensure safety and tolerance of finished cosmetic products.
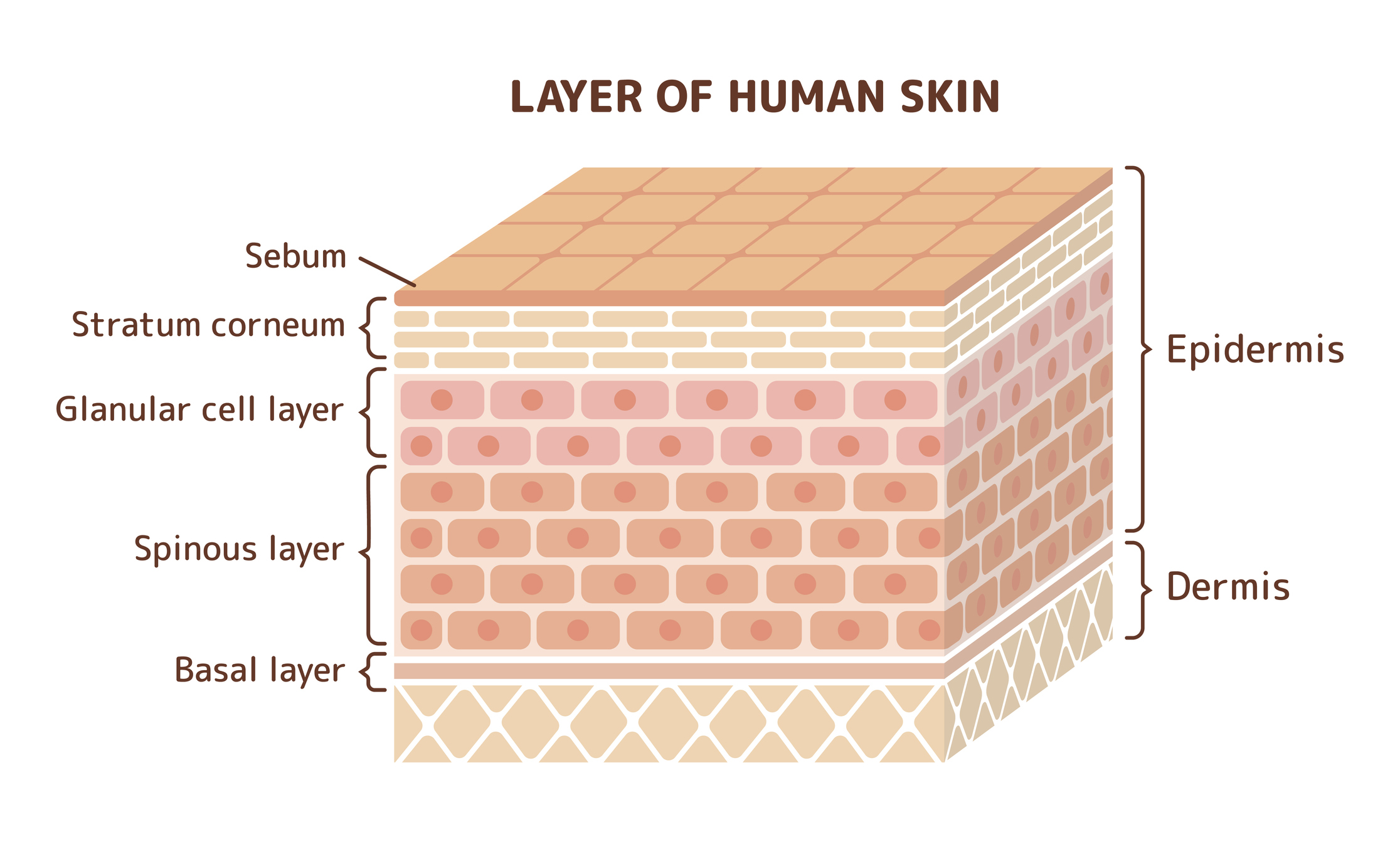
Evaluation of the demal absorption and delivery of a test substance using excised skin
In order to evaluate the possibility of peripheral effects during dermal administration of test compounds, it is essential to evaluate these compounds’ potential transcutaneous passage.
In vitro methods measure the diffusion of chemicals across skin into a fluid reservoir.
Cryopreserved skin is used to measure diffusion only, whereas fresh metabolically-active skin is used to simultaneously measure diffusion and skin metabolism.
Such methods have found particular usage as screenings tests for comparing transcutaneous delivery of chemicals from different formulations. These methods also provide useful models for the assessment of percutaneous absorption in humans. Acceptable data from a minimum of four replicates per test preparation are required.
Using appropriate conditions, which are described in the OECD 428 guidelines, absorption of test compounds during a given time interval is measured by analyzing both receptor fluid and the treated skin.
The test compound remaining on the skin’s surface should be considered as absorbed unless it can be conclusively demonstrated that absorption can be determined from receptor fluid values alone.
Analysis of other components (e.g. material washed off the skin or remaining within skin layers) allows for further data evaluation, including total test substance disposition and percentage recovery.
All components of the skin should be analyzed, and recovery should also be determined. This includes rinsing of the donor chamber, skin surface, and the receptor fluid/chamber.
In some cases, the skin may be fractionated into stratum corneum, epidermis, and dermis fractions for additional analysis.
Effect of the formulation on transcutaneous absorbtion of a test compound
Test of the efficacy of antimicrobial preservation in cosmetic products
Two methods are used:
- one according to European Pharmacopoeia,
- and another according to ISO 11930.
This test is performed by our specialist partner in microbiology.
Evaluation of potential irritation after application to the chorioallantoic membrane of embryonic hens’ eggs.
Published in the Official Journal of the French Republic on December 12, 1996.
Evaluation of potential irritation by direct application to rabbit cornea using the neutral red release method.
Published in the Official Journal of the French Republic on December 30, 1999.
Evaluation of ocular tolerance performed on 0.5cm² reconstructed corneal epithelium (HCE) obtained from human corneal epithelial cells.
Assay was developed based on guidelines related to ingredients and further adapted to finished products, in collaboration with Pierre Fabre. Assay is based on scientific data that measures correlation between classifications obtained during clinical tests and in vitro tests.
Advantages:
- Model displays greater resemblance to real human tissue, increasing its relevance and predictive power as related to normal physiology
- Model enables execution of more relevant and reliable tests, increasing its resemblance to usual conditions of product application
Method exclusively developed and used by Eurosafe for evaluation of potential irritation of human skin performed on fresh human skin discs.
Product effects on epidermis are evaluated using an MTT cell viability test.
Evaluation of cutaneous tolerance performed on reconstructed human epidermis (RHE) of 0.5 cm² obtained from normal human keratinocytes.
Assay was developed based on guidelines related to ingredients and further adapted to finished products, in collaboration with Pierre Fabre. Assay is based on scientific data that measures correlation between classifications obtained during clinical tests and in vitro tests.
Advantages:
- Model displays greater resemblance to real human tissue, increasing its relevance and predictive power as related to normal physiology
- Model enables execution of more relevant and reliable tests, increasing its resemblance to usual conditions of product application
Evaluation of tolerance performed on reconstructed vaginal epithelium (HVE) derived from squamous cell carcinoma of vulva.
Assay was developed based on guidelines related to ingredients and further adapted to finished products, in collaboration with Pierre Fabre. Assay is based on scientific data that measures correlation between classifications obtained during clinical tests and in vitro tests.
Advantages:
- Model displays greater resemblance to real human tissue, increasing its relevance and predictive power as related to normal physiology
- Model enables execution of more relevant and reliable tests, increasing its resemblance to usual conditions of product application
- Model provides solution for evaluation of personal hygiene products.
Evaluation of gingival tolerance performed on reconstructed gingival epithelium (HGE) of 0.5 cm² derived from transformed human gingival epithelial cells.
Assay was developed based on guidelines related to ingredients and further adapted to finished products, in collaboration with Pierre Fabre. Assay is based on scientific data that measures correlation between classifications obtained during clinical tests and in vitro tests.
Advantages:
- Model displays greater resemblance to real human tissue, increasing its relevance and predictive power as related to normal physiology
- Model enables execution of more relevant and reliable tests, increasing its resemblance to usual conditions of product application
- Model provides solution for evaluation of oral hygiene products.
Evaluation of potential cutaneous phototoxicity on reconstructed human epidermis (RHE) upon UVA exposure. The effects of the product on the epidermis are evaluated by an MTT cell viability test.
Assay was developed based on guidelines related to ingredients and further adapted to finished products, in collaboration with Pierre Fabre. Assay is based on scientific data that measures correlation between classifications obtained during clinical tests and in vitro tests.
Advantages:
- Model displays greater resemblance to real human tissue, increasing its relevance and predictive power as related to normal physiology
- Model enables execution of more relevant and reliable tests, increasing its resemblance to usual conditions of product application
- Model allows to meet the requirements of Vegan products
In vivo assays
Eurosafe evaluates tolerance and efficacy using our standardized assays or personalized protocols that are compliant with your needs. Our team is available to discuss and support you in all your development projects.
Cutaneous tolerance patch test under dermatological control with 20 volunteers 1 reading at 48h
We support different players in the cosmetics industry in diverse distribution channels.
Our goal is to offer you:
- competitive prices
- Flexibility and optimized delivery times
- Personalized follow-up
Our teams are at your disposal to discuss your projects in particular and to offer standardized or specific assays according to your specifications.
Our services :
standardized assays to evaluate tolerance and perceived efficay of your products
- Collaboration with medical specialists according to your needs (i.e. dermatologists, ophthalmologists, gynecologists, oral-maxillofacial surgeons, etc.)
- Subjective evaluation of efficacy using post-study surveys that measure percentage of satisfaction.
innovative tests to evaluate the efficacy of your products :
- Skin microbiome evaluation : determination by 16S metagenomic sequencing of the bacterial composition of cutaneous samples taken from volunteers of a use test. The goal is to conclude that the tested product does not imply a major modification of the microbiota .
database of volunteers with different phenotype and populations:
- Sensitive skin, mature skin, dry skin, damaged hair, lens wearers, sensitive mucous membranes ..,
- Caucasian and Asian subjects with tests conducted in Asia.
These solutions are compliant for all finished cosmetic products:
- Hygiene products, both rinsed and non-rinsed,
- Personal hygiene products,
- Care products
- Anti-wrinkle products,
- Slimming products,
- Deodorants,
- Oral products,
- Makeup...
and answer to your claims:
- Moisturizer,
- anti wrinkle,
- Firming,
- Sebum regulator,
- Deodorant effect,
- Slimming,
- Anti-tasks,
- Non-comedogenic...
Regulations require that any claimed effects must be supported by appropriate evidence, and prohibit false attribution of unverified features or functions to any products.
The efficacy of cosmetic products must be established from data collected from ingredients and from the finished product.
"Claims relating to cosmetic products, whether explicit or implicit, must be based on adequate and verifiable evidence, irrespective of their type..."
Extract from Commission Regulation (EU) No 655/2013 of 10 July 2013 which established common criteria related to cosmetic claims .
Our services :
Well-established methods to objectively measure your claims:
- Measured instruments:cutometry, corneometry, chromametry, glossymétry, sebumetry, impedance, centimeter measurements ...
- Self evaluation: evaluation conducted by volunteers using a visual analogical scale at inception and completion of research studies. Percentage change will be assessed for each parameter to be evaluated.
- Expert evaluation: wrinkles on photographic scale (Fotofinder Médiscope), sniff test, quantification of comedones,...
Specific methods
- 16S metagenomic sequencing for skin microbiome evaluation: determination of the bacterial composition of cutaneous samples taken from volunteers of a use test. The goal is to conclude that the tested product does not imply a major modification of the microbiota .
To meet your specifications, Eurosafe offers jointly-developed and customizable protocols for your cosmetic products.
Examples of personalized studies:
- consumption and exposure of solar products,
- consumption and exposure of oral products, etc.


